Background
Peripheral T-cell lymphomas (PTCLs) comprise a rare, heterogeneous group of non-Hodgkin lymphomas of mature T-cell origin. Most systemic (s) PTCL subtypes typically have poor outcomes with conventional anthracycline-based chemotherapy. Biomarker-driven and subtype-specific treatments are promising but unmet needs. Few benchmark studies exist to characterize the current real-world landscape of clinical and pathologic practice. We report the patterns of care and updated outcome data for PTCL patients enrolled in the LEO-MER multi-center prospective cohort study (ClinicalTrials.gov NCT02736357).
Methods
Patients aged 18 years or older with newly diagnosed PTCL were prospectively enrolled in the University of Iowa/Mayo Clinic MER cohort (2002-2015) or the expanded LEO cohort (2015-2020). Clinical, pathology, treatment, and outcome data were abstracted from medical records using a standard protocol in both cohorts. Pathology underwent expert re-review based on WHO criteria. Overall survival (OS) was calculated from date of diagnosis to date of death or last follow-up. Event-free survival (EFS) was calculated from date of diagnosis to disease progression, initiation of 2 nd line therapy, or death from any cause. EFS and OS were evaluated using Cox model and Kaplan-Meier estimator. Log-rank test was used to test the significance of difference between groups.
Results
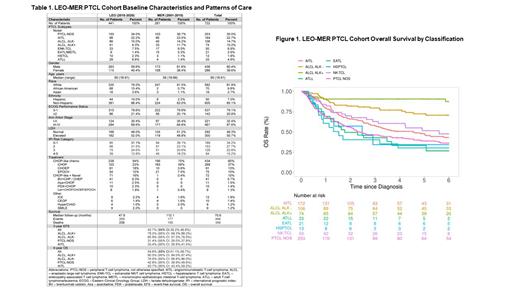
LEO-MER enrolled 1132 PTCL patients during the study period (462 MER and 670 LEO). Of these, 722 were sPTCLs (281 MER and 441 LEO), including PTCL-NOS (N=253, 35%), anaplastic large cell lymphoma (ALCL; N=180, 24.9% [74 ALK+, 106 ALK-]), angioimmunoblastic T-cell lymphoma (AITL, N=164, 22.7%), extranodal NK-TCL (N=50, 6.9%), adult T-cell leukemia/lymphoma (N=33, 4.6%), enteropathy-associated TCL (N=19, 2.6%), and hepatosplenic TCL (N=13, 1.8%) ( Table 1). Median age at diagnosis was 60 years. M:F ratio was 1.5. Comparing LEO to MER, African American population was 15.4% vs 0.7%, and Hispanic was 10% vs 2.2%. Clinical characteristics were comparable between MER and LEO overall: PS≥2, 20.9%; elevated LDH, 50.7%; stage III-IV, 67.6%; and IPI 2-5, 65.7%.
The most common 1 st line chemotherapy regimens overall were CHOP-based (N=506, 70%), including 60% receiving CHOP-like chemotherapy in both MER and LEO cohorts (CHOP [N=268, 37%], CHOEP [N=91, 13%] or EPOCH [N=75, 10%]), and 16% with CHOP-like in combination with novel agents in LEO cohort (BV+ [N=41, 9.3%], azacitidine+ [N=11, 2.5%], pralatrexate+ [N=10, 2.3%], lenalidomide+ [N=8, 1.9%]) ( Table 1). More patients received etoposide in LEO (40.3%) than in MER (20.3%). 74 patients (10.3%) underwent consolidative SCT following induction chemotherapy (71 auto and 3 allo). 65 patients (11.2%) received frontline therapy on clinical trials. At a median follow-up of 9.4 years in MER and 4 years in LEO, 177 (MER) and 269 (LEO) events, and 150 (MER) and 206 (LEO) deaths were observed, respectively. The 3-year EFS and 4-year OS estimates for sPTCL were 46.3% and 59.1% in MER, and 40.4% and 51.3% in LEO. EFS and OS correlated with IPI scores and differed by sPTCL subtypes ( Figure 1). ALCL had superior survival, with 3-year EFS and 4-year OS of 78.2% and 90.5% for ALK+, and 65.9% and 76.9% for ALK-. Non-ALCL subtypes including PTCL-NOS and AITL had inferior outcomes, with 3-year EFS and 4-year OS of 31.4% and 42.8% for PTCL-NOS, and 33.4% and 50.7% for AITL. In subset analysis, the addition of etoposide to CHOP chemotherapy as either CHOEP or EPOCH was associated with improved OS for ALK- ALCL (p=0.038, log-rank), but not for ALK+ ALCL (p=0.75) or non-ALCL subtypes of PTCL-NOS and AITL (p=0.88).
Conclusion
The LEO-MER study is the largest prospective cohort study of PTCL to date. Patterns of care in the LEO cohort begin to incorporate novel agents in frontline therapy. Outcomes continue to mature with longitudinal follow-up and ongoing accrual, which poise to shape benchmarks in the contemporary era. The lack of benefit of etoposide adding to CHOP induction and poor overall survival of non-ALCL subtypes underscores the unmet need of therapeutic breakthrough for non-ALCL frontline treatment, particularly through clinical trials with biomarker-guided approaches incorporating novel agents.
Disclosures
Ruan:BMS: Research Funding; Genentech: Research Funding; Daiichi Sankyo: Research Funding; Secura Bio: Consultancy; AstraZeneca: Consultancy, Research Funding. Bennani:Acrotech: Other: Advisory board; No personal compensation; Acrotech: Other: Scientific Advisory Committee, No personal compensation ; Affimed: Other: Advisory board; No personal compensation; Secura Bio: Other: Advisory board; No personal compensation; Kymera: Other: Advisory board; No personal compensation; Astellas Pharma: Other: Advisory board; No personal compensation. Allen:Seattle Genetics: Consultancy; Secura Bio: Consultancy; Kyowa Kirin: Consultancy; Daiichi Sankyo: Consultancy. Cohen:BMS/Celgene: Research Funding; Novartis: Research Funding; Genentech: Research Funding; BioInvent: Research Funding; Lam Therapeutics: Research Funding; Takeda,: Research Funding; ADCT: Consultancy; AstraZeneca: Consultancy, Research Funding; Abbvie: Consultancy; Janssen: Consultancy; BeiGene: Consultancy; Loxo/Lilly: Consultancy, Research Funding. Vega:Allogene: Research Funding; Geron: Research Funding. Casulo:Verastem: Research Funding; Abbvie: Consultancy; GenMab: Research Funding; Gilead Sciences: Research Funding; SecuraBio: Research Funding; Follicular Lymphoma Foundation: Other: Leadership role; Bristol Myers Squibb: Consultancy, Research Funding; Lymphoma Research Foundation: Other: Leadership Role; Genentech: Consultancy, Research Funding. Mehta-Shah:Ono Pharmaceuticals: Consultancy; Corvus Pharmaceuticals: Research Funding; Genentech: Consultancy; Celgene: Research Funding; Kyowa Hakko: Consultancy; Daiichi Sankyo: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; C4 Therapeutics: Consultancy, Research Funding; Bristol Myers-Squibb: Research Funding; Genentech/Roche: Research Funding; Innate Pharmaceuticals: Research Funding; Secura Bio/Verastem: Consultancy, Research Funding; Karyopharm Therapeutics: Consultancy; Janssen: Consultancy. Martin:AbbVie, AstraZeneca, Beigene, Epizyme, Genentech, Gilead, Janssen, Pepromene, Daiichi Sankyo: Consultancy. Maurer:Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Research Funding; Roche/Genentech: Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees, Research Funding. Kahl:BeiGene: Consultancy, Honoraria, Research Funding; Astra Zeneca: Consultancy, Honoraria, Research Funding; ADCT: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria; Genentech: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Genmab: Consultancy, Honoraria; BMS: Consultancy, Honoraria. Lossos:LRF: Membership on an entity's Board of Directors or advisory committees; NCI: Research Funding; Adaptive: Honoraria; NCI: Research Funding; University of Miami: Current Employment; BeiGene: Consultancy. Cerhan:BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; NanoString: Research Funding; Genmab: Research Funding; Protagonist: Other: Safety Monitoring Committee; Genentech: Research Funding. Flowers:4D: Research Funding; Eastern Cooperative Oncology Group: Research Funding; Xencor: Research Funding; Burroghs Wellcome Fund: Research Funding; Adaptimmune: Research Funding; Acerta: Research Funding; SeaGen: Consultancy; Ziopharm: Research Funding; Spectrum: Consultancy; Allogene: Research Funding; Morphosys: Research Funding; Pfizer: Research Funding; Novartis: Research Funding; Nektar: Research Funding; Cellectis: Research Funding; Guardant: Research Funding; Iovance: Research Funding; Jannsen Pharmaceuticals: Research Funding; Sanofi: Research Funding; Pharmacyclics: Research Funding; Amgen: Research Funding; Takeda: Research Funding; TG Therapeutics: Research Funding; Karyopharm: Consultancy; Gilead: Consultancy, Research Funding; Genmab: Consultancy; Genentech Roche: Consultancy, Research Funding; Denovo Biopharma: Consultancy; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Celgene: Consultancy, Research Funding; Beigene: Consultancy; Bayer: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; N-Power Medicine: Consultancy, Current holder of stock options in a privately-held company; Pharmacyclics Jansen: Consultancy; Kite: Research Funding; National Cancer Institute: Research Funding; V Foundation: Research Funding; CPRIT Scholar in Cancer Research: Research Funding; Cancer Prevention and Research Institute of Texas: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal